Some media outlets reported enthusiastically that, Lecanemab was recently approved by the European Medicines Agency (EMA) for the treatment of Alzheimer’s disease. However, I find the actual data underwhelming… Here’s why.
What is Lecanemab?
Lecanemab is a monoclonal antibody that binds to soluble Aβ protofibrils and reduces the accumulation of beta-amyloid. It is administered as an infusion every two weeks for 18 months. It could be available in Europe by mid-2025.
How effective is Lecanemab?
The Phase 3 study (November 2022) involving 1,795 patients with mild Alzheimer’s disease showed that, after 18 months, there was a modest reduction in the decline of cognitive and functional symptoms of dementia:
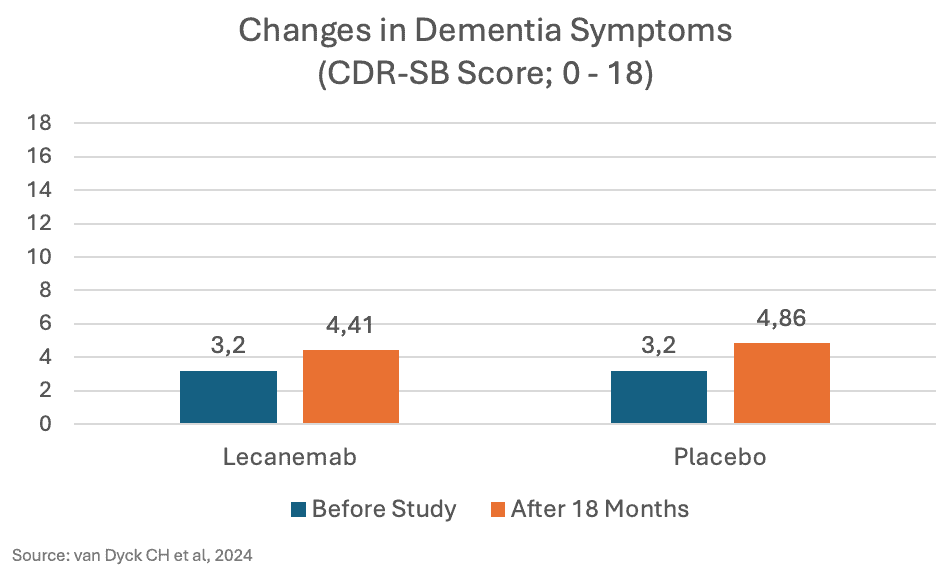
The difference of 0.45 points is very small. To put it more positively, the worsening of symptoms was reduced by 45% (from +1.21 to +1.66 CDR-SB points). However, for patients, the absolute difference is what matters.
At least the improvement in amyloid deposits seen in the PET scans was significant. A reduction of 55% was observed, compared to an increase of 4% in the placebo group.
Is this effect “clinically significant”?
That depends on how you define “clinically significant.” A recent review established threshold values for “minimal clinical important difference” in Alzheimer’s:
- Mild Cognitive Impairment (MCI-AD): Improvements of at least 1 point (CDR-SB) would be clinically significant.
- Mild Alzheimer’s Disease (mild AD): Improvements of at least 2 points (CDR-SB) would be clinically significant.
- A retrospective analysis of 20,000 patients showed that doctors typically perceive clinical improvement starting from a 0.98-point improvement (in MCI-AD) or 1.63 points (in mild AD).
The study mentioned above included patients with mild cognitive impairment or mild dementia due to Alzheimer’s. On average, they had 3.2 points (CDR-SB) at the start of the study. The observed difference of 0.45 points, based on these thresholds, is clinically not relevant—by a significant margin.
What is only mentioned in the appendix of the study…
..is that the effect was significantly lower in women than in men. The slowing of decline in activities of daily living (ADCS-MCI-ADL) was 54% in men and 19% in women; for the composite score (ADCOMS), it was 38% in men and 10% in women. This should be discussed more widely.
Why was Lecanemab initially rejected by the EMA, and then later approved?
Partly due to side effects. Deaths did not occur more frequently with Lecanemab. However, serious adverse events were more common with Lecanemab than with placebo (14.0% vs. 11.3%). Abnormal imaging findings (ARIA) with brain edema and brain hemorrhages occurred much more frequently (see graphic; but only 22% of the brain edemas observed in imaging were symptomatic). As homozygous ApoE4 gene copies (about 15% of patients) were identified as a risk factor, the EMA approval was granted only after excluding this patient group:
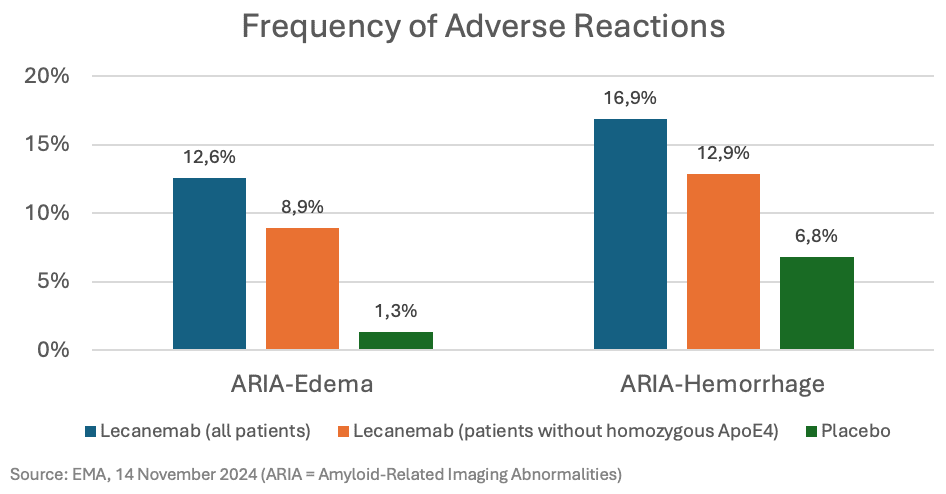
In July, the EMA decided that the benefits of Lecanemab did not outweigh the side effects (“blue”). In November, the EMA reversed its decision, stating that, after excluding patients with homozygous ApoE4, the benefits outweighed the risks (“orange”). However, not all regulatory bodies reached the same conclusion. In Australia, Lecanemab was rejected because its effectiveness was considered too small to justify the risks.
How expensive is the therapy?
Very expensive:
- In the US, the current cost is $26,500 per year.
- In the US, additional costs of $56,000 per year are incurred for genetic tests and MRIs.
- In the EU, up to 5.4 million people could receive the therapy; at American prices, this would amount to €133 billion per year (!)
These are high costs for a drug with an unclear benefit-risk profile. A study found that Lecanemab would only be cost-effective for mild dementia in certain scenarios if it cost under $5,100 per year.
Conclusion:
- The efficacy is low (0.45 points on a 0-18 scale; CDR-SB).
- This efficacy is lower than the proposed thresholds of 1 or 2 points for “clinical relevance.”
- This efficacy is even lower in women compared to men.
- Side effects (including brain edema and brain hemorrhages) are still common, even after excluding patients with homozygous ApoE4.
- The study lasted only 18 months. Long-term effects and side effects are still unknown.
- The costs are high, over $80,000 per year in the US.