Demand for new obesity medications like semaglutide (GLP-1 analogs) is high, but availability is low. So, which patients should get them first? What are the true benefits and side effects? Patients ask me these questions fairly often, so I know it’s important for GPs to know the key facts. I therefore reviewed the main research studies and guidelines and tried to summarize them briefly and clearly in this article.
1. How effective are GLP-1 analogs for weight loss?
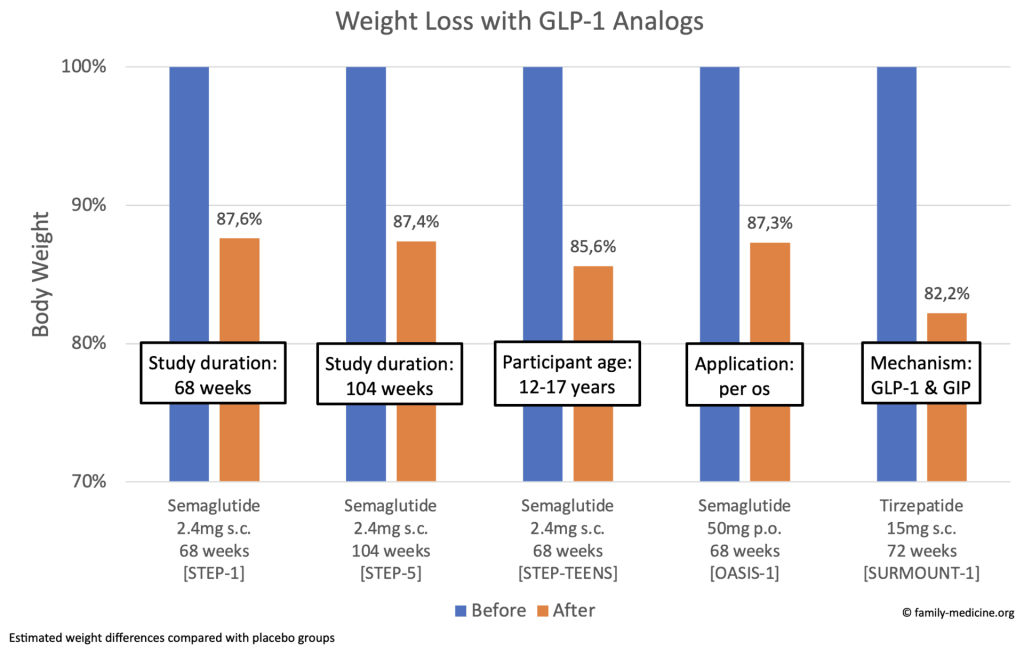
As you can see, the weight loss effects are significant and sustained. Semaglutide per os (50 mg) was as effective as subcutaneous application (2.4 mg). Tirzepatide (15 mg) showed slightly more noticeable effects than Semaglutide (2.4 mg).
Does the rebound effect really exist?
Yes, one year after stopping the medication, patients regain ½ – ⅔ of their previous weight loss:

What are other clinical benefits?
Cardiovascular Events
The SELECT trial1 randomized 17,604 patients with preexisting CVD and overweight/obesity. Relative risk for cardiovascular events was reduced by 19% (RRR), while the absolute risk was reduced by 1.5% over 40 months (ARR). In other words, you must treat 67 CVD patients for 40 months to avoid one such event (for CVD patients, NNT=67 for 40 months).

In March 2024, FDA therefore approved semaglutide for secondary CVD prevention in overweight or obese adults.2
Heart Failure
The STEP-HFpEF trial3 randomized 529 patients with heart failure (EF≥45%, BMI≥30) and treated them with s.c. semaglutide 2.4 mg or placebo for 52 weeks. Clinical benefits in patients with heart failure seem really promising:

The improvements in heart failure symptoms strongly correlate with the scale of weight reduction: those who lost <5% of their weight improved their KCCQ-CSS (heart failure score; 1-100) by only 5 points, while those who lost 15%-20% improved by 20 points.4
Chronic Kidney Disease
The FLOW trial randomized 3,534 patients with chronic kidney disease and type 2 diabetes and started treating them with s.c. semaglutide 1.0 mg or placebo in 2019. The study was terminated early because benefits were already significantly visible by October 2023.5 In March 2024, Novo Nordisk announced that 24% less CKD-related events occurred in the intervention group.6
2. How common are side effects related to semaglutide?
Discontinuation
Large trials show that around 4% of patients discontinue (due to adverse events, compared to placebo) after 16-17 months7,8 and around 8% quit after 34 months1:

GI Symptoms
Nausea, vomiting, diarrhea, constipation, and abdominal pain occur in >10% of patients. These symptoms are usually mild or moderate and short.9
Diabetic Retinopathy
This condition can newly occur or worsen, probably due to rapid improvement in glucose control:

In patients with diabetes, the decision becomes an issue of heart benefits vs. eye harm. Both the FDA and EMA recommend that doctors monitor patients with diabetic retinopathy who are undergoing semaglutide treatment closely.10,9 In patients with uncontrolled or potentially unstable diabetic retinopathy, treatment with semaglutide 2.4 mg is not recommended.9 The American Academy of Ophthalmology offers more information here11.
Pancreatitis
The EMA considers pancreatitis uncommon (0.2% of patients),9 but it still received some media coverage as a side effect, due to a study12 which suggested a ‘nine-fold’ increase. However, this cohort study was small, and the finding’s range was therefore very large, from a 25% to a 6,600% (!) increase (95% CI: 1.25 to 66.00). Interpreting this result as a ‘nine-fold’ increase neglects this uncertainty and is therefore not appropriate.
Thyroid Cancer
While the FDA included a warning for thyroid c-cell tumors and MEN-2,10 in October 2023 the EMA concluded “that the available evidence does not support a causal association” between GLP-1 agonists and thyroid cancers.13 Here is some evidence: From 2004-2020, 8,718 GLP-1 analogs-associated tumors were reported to the FDA.14 While the overall cancer risk was not increased, thyroid and pancreatic cancers were reported 7 and 10 times more often than with other drugs. However, keep in mind that at least part of this association might be explained by improved reporting due to previous warnings! In March 2024, this was supported by a large Scandinavian cohort study which followed 145,410 patients over four years and found no increased risk of thyroid cancer.15 Also, a systematic review (2019) of 50,452 diabetes patients did not show a statistically significant increase of the overall cancer risk (OR 1.04; 95% CI: 0.94 to 1.15).16
Suicidal Thoughts
Recently, suicidal ideation was investigated as a potential GLP-1 agonist side effect. Overall, EMA received 150 such reports after an exposure of over 20 million patient years.17 An analysis of 1.8 million electronic health records in the USA showed a lower risk for suicidal thoughts in those taking semaglutide than in those taking other weight loss medications.18 While the EMA did not list suicidal behavior or ideation as a side effect,17 the FDA recommends monitoring and discontinuing semaglutide if such symptoms develop.10
3. Conclusions
What do guidelines recommend?
Because of the global shortage, many countries recommend you do not initiate new patients on semaglutide. However, this advice may change soon.
The only more specific guideline I could find on semaglutide for weight loss is from NICE in the UK (September 2023).19 It states that only new patients who met the following criteria could get semaglutide:
- Obesity and at least one weight-related complication (more details in the guideline)
- Patients within a multidisciplinary, specialist weight management service
- Maximum of 2 years
- Stop if <5% weight loss after 6 months
My Thoughts
New GLP-1 analogs for obesity seem to be highly effective for weight loss, but also for reducing cardiovascular risk and for improving heart failure symptoms. While side effects are relevant, many patients could still benefit a lot from the drug. However, these questions seem very important to me:
- How do we prioritize patients when demand is high and availability is low? Some countries apply a first-come-first-served principle: Those already on GLP-1 analogs should continue; others should wait. But wouldn’t some obese patients at high cardiovascular risk or with heart failure benefit more?
- What happens after two years of treatment? Do we continue without evidence of long-term studies, or discontinue with evidence of a large rebound effect?
- Should everyone who could benefit get a prescription? Everyone with obesity or CVD (that’s more than 10% of our population)? At the same time, should we continue to allow fast food to be sold at schools? It’s great to have effective medicine against obesity, but in the end, we’re treating the symptoms of an unhealthy food industry. As a doctor and also a public health researcher, I would appreciate if we take preventative action to tackle the root of the problem as well.
Summary
- GLP-1 analogs show significant weight loss in obese adults and adolescents (around 13%-18%).
- This effect lasts for (at least) two years while taking the medication (longer studies are not available yet).
- Stopping the medication leads to a rebound effect (½ – ⅔ weight regain after one year).
- Patients with CVD are at lower risk for cardiovascular events (19% relative risk reduction, NNT of 67 over 40 months).
- Patients with heart failure show less hospital admissions improved symptoms (in correlation to their weight loss).
- Patients with CKD experience less CKD-related events (24% less).
- Minor side effects are common (especially gastrointestinal); major side effects are probably uncommon.
- In the future, oral application might also be available.